
Scope of Services
From consulting and strategy development to implementation and support, our comprehensive services can help your business thrive.

Effective site management is crucial for the success of clinical trials. It ensures that site teams conduct trials efficiently, adhering to protocols, Good Clinical Practice (GCP) standards, and relevant regulations. This approach is essential for maintaining data accuracy and safeguarding participants.
Site Management Organization



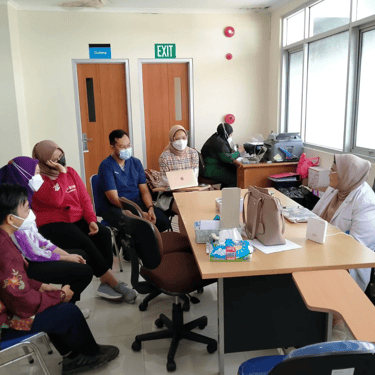


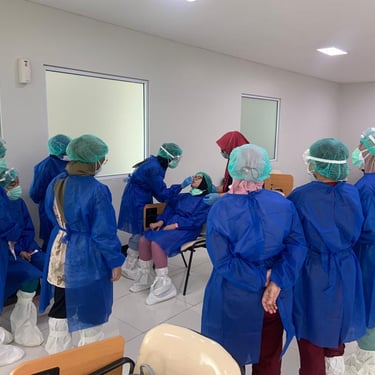

Feasibility Assessments
Streamlining Clinical Trials: Bridging Sites and Patients
Protocol Development
Site and Investigator Selection
Regulatory Compliance

Protocol Development
Your Partner in Developing Robust Clinical Trial Protocols
At Yayasan RPRI, we understand that a meticulously crafted protocol is the cornerstone of successful clinical research. Our protocol development service is designed to guide investigators and sponsors through the intricate process of creating comprehensive, scientifically rigorous, and regulatory-compliant protocols.

Project Implementation



Project Implementation at RPRI: Partnering for Progress
Effective project implementation in healthcare requires adequate resources, expertise, and experience. Many organizations struggle with selecting the right methodologies due to limited references.


Take Action, No Discrimination!
Harnessing social media to deliver health education and promotion for community & make community engagement for eradicating Tuberculosis.
Tuberculosis TV

Unlocking an Indonesia free of TB and respiratory problems

Head Office Address
Jl. Balai Pustaka Barat 358E
Rawamangun, Pulo Gadung
Jakarta Timur, 13220
Any questions and inquiries, Feel free to contact us:


Contact Us
Corresponding Address
Jalan Pegambiran No. 37 lantai 2,
Rawamangun, Pulo Gadung
Jakarta Timur, 13220






